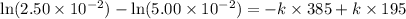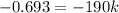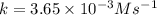Chemistry, 22.04.2020 22:57 grayjasmine46
The reactant concentration in a zero-order reaction was 5.00×10−2 M after 195 s and 2.50×10−2 M after 385 s . What is the rate constant for this reaction? Express your answer with the appropriate units. Indicate the multiplication of units, as necessary, explicitly either with a multiplication dot or a dash.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1


Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2
You know the right answer?
The reactant concentration in a zero-order reaction was 5.00×10−2 M after 195 s and 2.50×10−2 M afte...
Questions












Biology, 17.10.2019 05:00








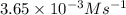
![\ln [A]=-kt+\ln [A_o]](/tpl/images/0619/6365/bdc3f.png)
![[A_o]](/tpl/images/0619/6365/dc622.png) = initial concentration
= initial concentration![[A]](/tpl/images/0619/6365/6aa06.png) = final concentration =
= final concentration = 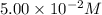 at 195 s
at 195 s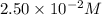 at 385 s
at 385 s![\ln (5.00\times 10^{-2})=-k\times 195+\ln [A_o]](/tpl/images/0619/6365/3a318.png) ............(1)
............(1)![\ln (2.50\times 10^{-2})=-k\times 385+\ln [A_o]](/tpl/images/0619/6365/497e4.png) ............(2)
............(2)![\ln (2.50\times 10^{-2})-\ln (5.00\times 10^{-2})=-k\times 385+\ln [A_o]+k\times 195-\ln [A_o]](/tpl/images/0619/6365/4b64f.png)