
Chemistry, 25.04.2020 01:25 scarbroughmary0
Environmental studies usually involve an analysis of precipitation and its response to pollution. To quantify the degree of contamination in natural rain water or snow, titration is used. The process is quick and results are reliable. Since most titration processes do not require expensive or specialized equipment, the test can be performed often and in different areas with relatively little effort. a. A 1000.0 mL sample of lake water is titrated using 0.100 mL of a 0.100 M base solution. What is the molarity of the acid in the lake water? Show all work. b. Based on the molarity of the acid calculated above, what is the pH of the lake water?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3
You know the right answer?
Environmental studies usually involve an analysis of precipitation and its response to pollution. To...
Questions


Mathematics, 03.09.2020 21:01




English, 03.09.2020 21:01


History, 03.09.2020 21:01

English, 03.09.2020 21:01


Mathematics, 03.09.2020 21:01

History, 03.09.2020 21:01



Mathematics, 03.09.2020 21:01

English, 03.09.2020 21:01




Chemistry, 03.09.2020 21:01

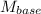 = 0.1 M
= 0.1 M
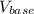 = 0.1 ml or 0.0001 litre
= 0.1 ml or 0.0001 litre
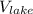 = 1000ml or 1 litre
= 1000ml or 1 litre
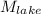 = ?
= ?

![pH=-log[H^+]\\\\pH=-log[0.00001]\\\\pH=5](/tpl/images/0626/1757/8eb3f.png)
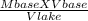
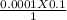
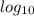 [
[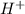 ]
]

