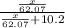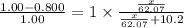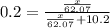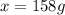Chemistry, 25.04.2020 04:06 amortegaa805
What mass of ethylene glycol, when mixed with 183 g H2O, will reduce the equilibrium vapor pressure of H2O from 1.00 atm to 0.800 atm at 100 °C? The molar masses of water and ethylene glycol are 18.02 g/mol and 62.07 g/mol, respectively. Assume ideal behavior for the solution.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2

Chemistry, 22.06.2019 17:20
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3
You know the right answer?
What mass of ethylene glycol, when mixed with 183 g H2O, will reduce the equilibrium vapor pressure...
Questions

English, 15.07.2019 11:00

Social Studies, 15.07.2019 11:00


Biology, 15.07.2019 11:00

English, 15.07.2019 11:00


Business, 15.07.2019 11:00




Chemistry, 15.07.2019 11:00

Biology, 15.07.2019 11:00

Mathematics, 15.07.2019 11:00



Mathematics, 15.07.2019 11:00


Mathematics, 15.07.2019 11:00


Geography, 15.07.2019 11:00

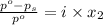
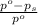 = relative lowering in vapor pressure
= relative lowering in vapor pressure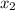 = mole fraction of solute =
= mole fraction of solute =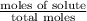
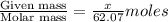
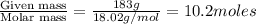
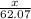 + 10.2
+ 10.2