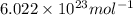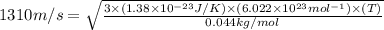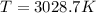Suppose that the root‑mean‑square velocity vrms of carbon dioxide molecules (molecular mass is equal to 44.0 g/mol ) in a flame is found to be 1310 m/s. What temperature T does this represent? The Boltzmann constant is k=1.38×10−23 J/K and Avogadro's number is A=6.022×1023 mol−1.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3

Chemistry, 22.06.2019 01:30
Asap! how do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 1

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 12:30
Nebulae are enormous clouds in outer space. they are made mostly of hydrogen gas, helium gas, and dust. some nebulae glow brightly, while others do not. the stars that people see are huge, bright balls of glowing gas. they are made mostly of hydrogen and helium. which statement correctly describes other ways in which nebulae and stars are different? a. stars can form inside a nebula but a nebula can never be produced by any star. b. a star always has a higher density than a nebula. c. stars can never form inside a nebula but a nebula can be produced by any star. d. a nebula always has a higher density than a star.
Answers: 3
You know the right answer?
Suppose that the root‑mean‑square velocity vrms of carbon dioxide molecules (molecular mass is equal...
Questions




Mathematics, 02.04.2020 02:39

Chemistry, 02.04.2020 02:39




Chemistry, 02.04.2020 02:39

Computers and Technology, 02.04.2020 02:39



Physics, 02.04.2020 02:39

Mathematics, 02.04.2020 02:39


Mathematics, 02.04.2020 02:39

Mathematics, 02.04.2020 02:39



World Languages, 02.04.2020 02:39

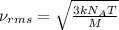
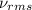 = root mean square speed = 1310 m/s
= root mean square speed = 1310 m/s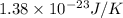
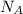 = Avogadro’s number =
= Avogadro’s number =