
Chemistry, 06.05.2020 07:41 punkinrichard1oxon2i
Suppose 0.829g of zinc chloride is dissolved in 100.mL of a 0.60M aqueous solution of potassium carbonate. Calculate the final molarity of chloride anion in the solution. You can assume the volume of the solution doesn't change when the zinc chloride is dissolved in it. Be sure your answer has the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1

You know the right answer?
Suppose 0.829g of zinc chloride is dissolved in 100.mL of a 0.60M aqueous solution of potassium carb...
Questions

Physics, 13.07.2020 20:01




Mathematics, 13.07.2020 20:01



Mathematics, 13.07.2020 20:01


Mathematics, 13.07.2020 20:01






Mathematics, 13.07.2020 20:01

English, 13.07.2020 20:01

Mathematics, 13.07.2020 20:01

Geography, 13.07.2020 20:01

Biology, 13.07.2020 20:01

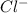 anion in solution is 0.122 M.
anion in solution is 0.122 M.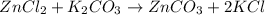
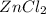 .
.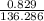 moles of
moles of 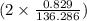 moles
moles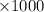
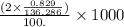 M
M

