
Chemistry, 15.10.2019 18:40 dylankrenek
Butane, c4h10, reacts with oxygen, o2, to form water, h2o, and carbon dioxide, co2, as shown in the following chemical equation: 2c4h10(g)+13o2(g)-> 10h2o(g)+8co2(g)
calculate the mass of water produced when 1.77 grams of butane reacts with excessive oxygen?
calculate the mass of butane needed to produce 71.6 of carbon dioxide.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 22.06.2019 23:00
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3

Chemistry, 23.06.2019 00:10
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3

Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1
You know the right answer?
Butane, c4h10, reacts with oxygen, o2, to form water, h2o, and carbon dioxide, co2, as shown in the...
Questions





Chemistry, 16.12.2020 04:40

Mathematics, 16.12.2020 04:40

Advanced Placement (AP), 16.12.2020 04:40

Mathematics, 16.12.2020 04:40

Biology, 16.12.2020 04:40

Mathematics, 16.12.2020 04:40

Computers and Technology, 16.12.2020 04:40





Mathematics, 16.12.2020 04:40


Health, 16.12.2020 04:40

Biology, 16.12.2020 04:40

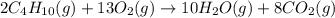
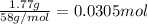
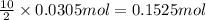 of water
of water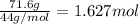
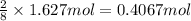 of butane
of butane

