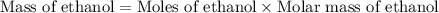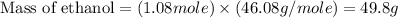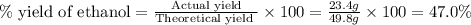Glucose (C6H12O6)(C6H12O6) can be fermented to yield ethanol (CH3CH2OH)(CH3CH2OH) and carbon dioxide (CO2).
C6H12O6⟶2CH3CH2OH+2CO2
The molar mass of glucose is 180.15 g/mol,180.15 g/mol, the molar mass of ethanol is 46.08 g/mol,46.08 g/mol, and the molar mass of carbon dioxide is 44.01 g/mol.
a) What is the theoretical yield (in grams) of ethanol from the fermentation of 97.5 g of glucose?
b) If the reaction produced 23.4 g of ethanol, what was the percent yield?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2

Chemistry, 23.06.2019 00:20
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1

Chemistry, 23.06.2019 05:50
Which of the following is not a characteristic of s waves?
Answers: 1
You know the right answer?
Glucose (C6H12O6)(C6H12O6) can be fermented to yield ethanol (CH3CH2OH)(CH3CH2OH) and carbon dioxide...
Questions

History, 16.06.2020 05:57


Mathematics, 16.06.2020 05:57




Mathematics, 16.06.2020 05:57

Mathematics, 16.06.2020 05:57

Spanish, 16.06.2020 05:57


Mathematics, 16.06.2020 05:57


Mathematics, 16.06.2020 05:57


Physics, 16.06.2020 05:57

Mathematics, 16.06.2020 05:57

Arts, 16.06.2020 05:57

Mathematics, 16.06.2020 05:57

Mathematics, 16.06.2020 05:57

Mathematics, 16.06.2020 05:57

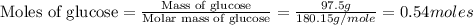
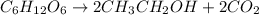
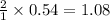 mole of ethanol
mole of ethanol