

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2


Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1
You know the right answer?
For the reaction shown, calculate how many moles of NH3 form when 16.72 moles of reactant completely...
Questions

Mathematics, 01.06.2021 15:20




Social Studies, 01.06.2021 15:20

Mathematics, 01.06.2021 15:20

Mathematics, 01.06.2021 15:20

Mathematics, 01.06.2021 15:20

Social Studies, 01.06.2021 15:20


English, 01.06.2021 15:20



Business, 01.06.2021 15:20


Physics, 01.06.2021 15:20

Chemistry, 01.06.2021 15:20



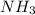 formed are, 22.3 moles.
formed are, 22.3 moles.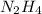 = 16.72 mol
= 16.72 mol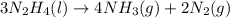
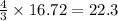 moles of
moles of 

