
Chemistry, 05.05.2020 08:19 iwannabewinston
Part A - Which numbers are used? Consider the balanced chemical equation that follows. You are asked to determine how many moles of water you can produce from 4.0 molmol of hydrogen and excess oxygen. (Excess oxygen means that so much oxygen is available it will not run out.) Which of the numbers that appear in the balanced chemical equation below are used to perform this calculation? 2H2(g)+O2(g)→2H2O(l)2H2(g)+O2(g)→2H 2O(l) View Available Hint(s)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1


Chemistry, 23.06.2019 07:00
Scuba divers use tanks of compressed air to them breathe. gases can be compressed because?
Answers: 1

Chemistry, 23.06.2019 11:00
Nh4no3 n2o + 2h2o a chemist who is performing this reaction starts with 160.1 g of nh4no3. the molar mass of nh4no3 is 80.03 g/mol; the molar mass of water (h2o) is 18.01 g/mol. what mass, in grams, of h2o is produced?
Answers: 1
You know the right answer?
Part A - Which numbers are used? Consider the balanced chemical equation that follows. You are asked...
Questions

History, 16.11.2019 13:31





History, 16.11.2019 13:31


Mathematics, 16.11.2019 13:31

Mathematics, 16.11.2019 13:31

Health, 16.11.2019 13:31




Mathematics, 16.11.2019 13:31

Biology, 16.11.2019 13:31

Mathematics, 16.11.2019 13:31

Health, 16.11.2019 13:31

English, 16.11.2019 13:31

Mathematics, 16.11.2019 13:31

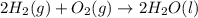
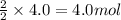 of water.
of water.

