Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:30
A48 g piece of ice at 0.0 ∘c is added to a sample of water at 7.4 ∘c. all of the ice melts and the temperature of the water decreases to 0.0 ∘c. how many grams of water were in the sample?
Answers: 1

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2
You know the right answer?
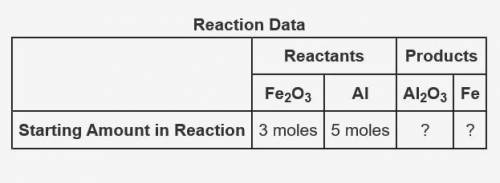
The following data was collected when a reaction was performed experimentally in the laboratory.
Questions


English, 26.11.2021 20:00


Chemistry, 26.11.2021 20:10

Geography, 26.11.2021 20:10


Social Studies, 26.11.2021 20:10

History, 26.11.2021 20:10




Mathematics, 26.11.2021 20:10

Mathematics, 26.11.2021 20:10

Mathematics, 26.11.2021 20:10

History, 26.11.2021 20:10