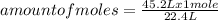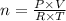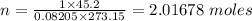Chemistry, 05.05.2020 01:36 Carrchris021
A container contains 45.2 L of N2 gas at STP. How many moles of N2 gas are in the container?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1
You know the right answer?
A container contains 45.2 L of N2 gas at STP. How many moles of N2 gas are in the container?...
Questions

Mathematics, 27.06.2019 09:30


Business, 27.06.2019 09:30



Mathematics, 27.06.2019 09:30

Health, 27.06.2019 09:30


Mathematics, 27.06.2019 09:30



Mathematics, 27.06.2019 09:30

Mathematics, 27.06.2019 09:30

World Languages, 27.06.2019 09:30

Mathematics, 27.06.2019 09:30



Computers and Technology, 27.06.2019 09:30

Mathematics, 27.06.2019 09:30

Mathematics, 27.06.2019 09:30