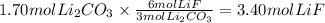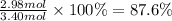The chemical reaction between lithium carbonate and aluminum trifluoride produces aluminum carbonate and lithium fluoride:
2AlF3 + 3Li2CO3 → Al2(CO3)3 + 6LiF.
You have an excess of aluminum trifluoride and 1.70 moles of lithium carbonate, which produces 2.98 moles of lithium fluoride. What is the percent yield of the reaction? Use the periodic table and this polyatomic ion resource.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1
You know the right answer?
The chemical reaction between lithium carbonate and aluminum trifluoride produces aluminum carbonate...
Questions