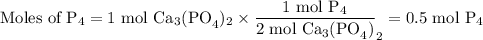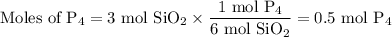Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3


Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3
You know the right answer?
How many moles of P4 will form when 1 mole of Ca3(PO4)2 reacts with 3 moles of SiO2 and 2 moles of C...
Questions



Physics, 12.11.2019 21:31



Mathematics, 12.11.2019 21:31


Health, 12.11.2019 21:31



Mathematics, 12.11.2019 21:31



Mathematics, 12.11.2019 21:31


Social Studies, 12.11.2019 21:31



Geography, 12.11.2019 21:31

Mathematics, 12.11.2019 21:31