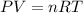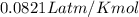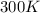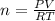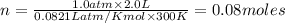Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:40
You may expect bonds between two atoms which each have n covalent lonic metallic hydrogen
Answers: 2


Chemistry, 22.06.2019 11:40
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3

Chemistry, 22.06.2019 16:50
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3
You know the right answer?
If the pressure of a gas is 1.0 atm, the volume is 2.0 L and the temperature is 300 k, how many mols...
Questions

Business, 09.01.2021 09:00


Business, 09.01.2021 09:00

Mathematics, 09.01.2021 09:00



History, 09.01.2021 09:00







Mathematics, 09.01.2021 09:00


Spanish, 09.01.2021 09:00

Biology, 09.01.2021 09:00


Business, 09.01.2021 09:00