Chemistry, 07.05.2020 02:13 montgomerykarloxc24x
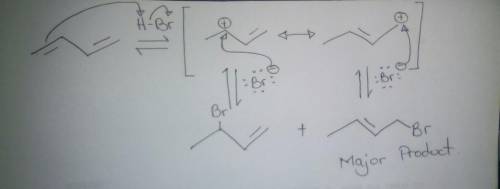
Draw the major product expected when 1,3-butadiene is treated with one equivalent of HBr at 40ºC and show the mechanism of its formation. For the mechanism, include lone pairs and charges in your answer. Do not draw out any hydrogen explicitly. Do not use abbreviations such as Me or Ph. A) Draw step 1 of the mechanism.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2
You know the right answer?
Draw the major product expected when 1,3-butadiene is treated with one equivalent of HBr at 40ºC and...
Questions

Computers and Technology, 09.12.2020 17:30

Mathematics, 09.12.2020 17:40


Business, 09.12.2020 17:40







Mathematics, 09.12.2020 17:40


Social Studies, 09.12.2020 17:40




English, 09.12.2020 17:40



Mathematics, 09.12.2020 17:40