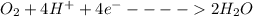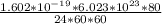Simply by living, an 87.5 kg human being will consume approximately 20.0 mol of O 2 per day. To provide energy for the human, the O 2 is reduced to H 2 O duing food oxidation by the reaction O 2 + 4 H + + 4 e − − ⇀ ↽ − 2 H 2 O Determine the current generated by the human per day. In this case, the current is defined as the flow of electrons ( e − ) to O 2 from the food the human consumes.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1


Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2
You know the right answer?
Simply by living, an 87.5 kg human being will consume approximately 20.0 mol of O 2 per day. To prov...
Questions

Chemistry, 06.01.2020 00:31


Mathematics, 06.01.2020 00:31


Computers and Technology, 06.01.2020 00:31

Mathematics, 06.01.2020 00:31


History, 06.01.2020 00:31



Chemistry, 06.01.2020 00:31

Arts, 06.01.2020 00:31

Mathematics, 06.01.2020 00:31

Mathematics, 06.01.2020 00:31


Mathematics, 06.01.2020 00:31

Mathematics, 06.01.2020 00:31



Social Studies, 06.01.2020 00:31