
Chemistry, 19.05.2020 22:17 12martinkat
Nswer the following questions relating to HCl, CH3Cl, and CH3Br. HCl(g), can be prepared by the reaction of concentrated H2SO4(ag), with NaCl(s), asa. represented by the following equation. H2SO4(ag) + 2 NaCl(s) → 2 HCl(g) Na2SO4(ag)A student claims that the reaction is a redox reaction. Is the student correct?i. Justify your answer. Calculate the mass, in grams, of NaCl(s), needed to react with excess H2SO4(ag)ii. to produce 3.0 grams of HCl(g). Assume that the reaction goes to completion

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 19:30
Astudent conducts an experiment to determine how the amount of water given to a plant affects its growth. what is the independent variable for this experiment?
Answers: 1

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1
You know the right answer?
Nswer the following questions relating to HCl, CH3Cl, and CH3Br. HCl(g), can be prepared by the reac...
Questions





Mathematics, 02.07.2020 03:01















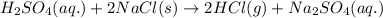
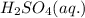 remain in excess amount therefore NaCl (s) is the limiting reagent. Hence production of HCl entirely depends on amount of NaCl used.
remain in excess amount therefore NaCl (s) is the limiting reagent. Hence production of HCl entirely depends on amount of NaCl used.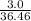 mol of HCl = 0.082 mol of HCl
mol of HCl = 0.082 mol of HCl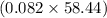 g = 4.8 g
g = 4.8 g

