Chemistry, 22.05.2020 06:58 josephvcarter
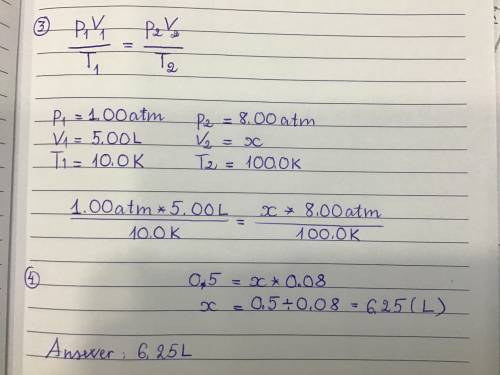
An ideal gas has a volume of 5.00L under a pressure of 1.00 atm and a temperature of 10.0 K. If the temperature is changed to 100.0 K while the pressure is increased to 8.00 atm what would be the new volume of the gas?
6.25L
1.60L
400.0L
16.0L

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3
You know the right answer?
An ideal gas has a volume of 5.00L under a pressure of 1.00 atm and a temperature of 10.0 K. If the...
Questions





Chemistry, 28.01.2021 08:30




Geography, 28.01.2021 08:30

Mathematics, 28.01.2021 08:30


History, 28.01.2021 08:30

Biology, 28.01.2021 08:30

Chemistry, 28.01.2021 08:30





Biology, 28.01.2021 08:30

Geography, 28.01.2021 08:30