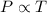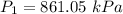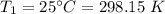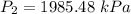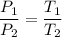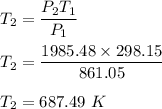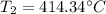Chemistry, 22.05.2020 23:57 naimareiad
2. A sample of gas has a pressure of 861.05 kPa at a temperature of 25°C. What temperature is required to
increase the pressure to 1985.48 kPa?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

You know the right answer?
2. A sample of gas has a pressure of 861.05 kPa at a temperature of 25°C. What temperature is requir...
Questions

Chemistry, 13.10.2019 17:30

Mathematics, 13.10.2019 17:30



English, 13.10.2019 17:30


Mathematics, 13.10.2019 17:30

Mathematics, 13.10.2019 17:30

Mathematics, 13.10.2019 17:30

Mathematics, 13.10.2019 17:30





English, 13.10.2019 17:30

Mathematics, 13.10.2019 17:30



Social Studies, 13.10.2019 17:30

Mathematics, 13.10.2019 17:30