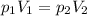A 2.00 L container of nitrogen had a pressure of 3.20 atm. What volume would be
necessary to d...


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1

Chemistry, 23.06.2019 09:20
Due tomorrow which would have a lower ph, a 0.1 m solution of a strong base or a weak base? why? which would have a higher ph, a 0.1 m solution of a strong base or a weak base? why?
Answers: 3
You know the right answer?
Questions


English, 03.07.2019 19:00

History, 03.07.2019 19:00


English, 03.07.2019 19:00

Computers and Technology, 03.07.2019 19:00

History, 03.07.2019 19:00




Mathematics, 03.07.2019 19:00

Mathematics, 03.07.2019 19:00

Arts, 03.07.2019 19:00






Biology, 03.07.2019 19:00

Physics, 03.07.2019 19:00