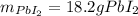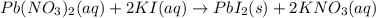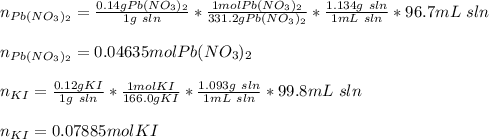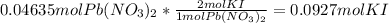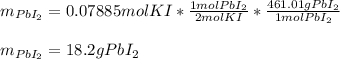Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:00
When light strikes a plane mirror, images form in locations where light does not actually reach. it only appears to the observer as though the light were coming from this position. what type of image is formed?
Answers: 2

Chemistry, 22.06.2019 04:30
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 07:00
How far is the region from the equator or control climate
Answers: 1
You know the right answer?
1. A 99.8 mL sample of a solution that is 12.0% KI by mass (d: 1.093 g/mL) is added to 96.7 mL of an...
Questions

Social Studies, 26.09.2019 23:40





Mathematics, 26.09.2019 23:40


Social Studies, 26.09.2019 23:40

Social Studies, 26.09.2019 23:40

Health, 26.09.2019 23:40






Mathematics, 26.09.2019 23:40


History, 26.09.2019 23:40


Mathematics, 26.09.2019 23:40