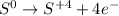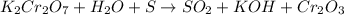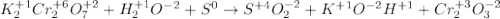Chemistry, 02.06.2020 21:00 cookiem0nster
Which is the correct oxidation half reaction for the following reaction K2Cr2O7 + H2O + S à SO2 + KOH + Cr2O3

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which element in the third period would you expect to have the larger atomic radius, sodium (na) or sulfur (s)? a. sodium, because it has a higher effective nuclear charge attracting electrons in fewer energy levels. b. sodium, because it has fewer protons attracting electrons in the same energy levels. c. sulfur, because it has more protons attracting electrons in more energy levels. d. sulfur, because it has a higher effective nuclear charge attracting electrons in the same energy levels.
Answers: 2

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2
You know the right answer?
Which is the correct oxidation half reaction for the following reaction K2Cr2O7 + H2O + S à SO2 + KO...
Questions

Mathematics, 19.11.2020 23:20



Mathematics, 19.11.2020 23:20


English, 19.11.2020 23:20

Mathematics, 19.11.2020 23:20


History, 19.11.2020 23:20



Mathematics, 19.11.2020 23:20


Biology, 19.11.2020 23:20

Mathematics, 19.11.2020 23:20

Mathematics, 19.11.2020 23:20


Spanish, 19.11.2020 23:20

Biology, 19.11.2020 23:20

Mathematics, 19.11.2020 23:20