
A chemist prepares a solution of iron(III) bromide (FeBr3) by measuring out 41.1 mg of FeBr3 into a 50. mL volumetric flask and filling to the mark with
distilled water.
Calculate the molarity of Br- anions in the chemist's solution
Be sure your answer is rounded to 2 significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2


Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3
You know the right answer?
A chemist prepares a solution of iron(III) bromide (FeBr3) by measuring out 41.1 mg of FeBr3 into a...
Questions


English, 22.03.2020 06:19

Business, 22.03.2020 06:20


Spanish, 22.03.2020 06:20


Physics, 22.03.2020 06:21

History, 22.03.2020 06:21


Mathematics, 22.03.2020 06:21


Mathematics, 22.03.2020 06:22

Mathematics, 22.03.2020 06:22


History, 22.03.2020 06:23

English, 22.03.2020 06:24

Mathematics, 22.03.2020 06:24



Mathematics, 22.03.2020 06:24

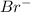 anions in the chemist's solution is 0.0084 M
anions in the chemist's solution is 0.0084 M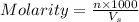
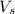 = volume of solution in ml
= volume of solution in ml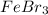 =
= 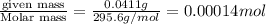
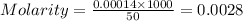
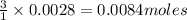 of
of 

