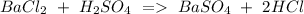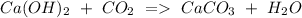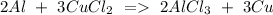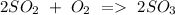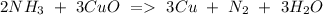Balance the following chemical equations.
a) Ba Cl2 + H2SO4 BaSO4 + HCl.
b) Calcium hy...

Chemistry, 13.06.2020 11:57 flyingcerberus1408
Balance the following chemical equations.
a) Ba Cl2 + H2SO4 BaSO4 + HCl.
b) Calcium hydroxide + Carbon dioxide Calcium carbonate + Water.
c) Aluminum + Copper chloride Copper + Aluminum chloride
d) Sulphur dioxide + Oxygen Sulphur trioxide
e) NH3+ CuO Cu + N2 + H2O

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:50
Which statement describes how phase changes can be diagrammed as a substance is heated? the phase is on the y-axis and the temperature is on the x-axis. the temperature is on the y-axis and the phase is on the x-axis. the time is on the y-axis and the temperature is on the x-axis. the temperature is on the y-axis and the time is on the x-axis.
Answers: 1

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1
You know the right answer?
Questions

Mathematics, 18.06.2020 20:57

Mathematics, 18.06.2020 20:57




Mathematics, 18.06.2020 20:57

Mathematics, 18.06.2020 20:57









Mathematics, 18.06.2020 20:57




History, 18.06.2020 20:57