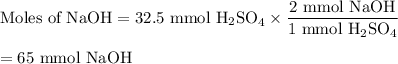Chemistry, 15.06.2020 01:57 gigimasters71p7tc6l
In a titration 32.5 mL of 1.0 M sulfuric acid is required to neutralize 45.0 mL of sodium hydroxide. What is the concentration of the base?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
If you want to create an electrical current, which situation would produce a solution capable of this
Answers: 3

Chemistry, 22.06.2019 00:20
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1
You know the right answer?
In a titration 32.5 mL of 1.0 M sulfuric acid is required to neutralize 45.0 mL of sodium hydroxide....
Questions


History, 27.06.2019 03:30


Mathematics, 27.06.2019 03:30


Mathematics, 27.06.2019 03:30





Mathematics, 27.06.2019 03:30


Mathematics, 27.06.2019 03:30






Mathematics, 27.06.2019 03:30