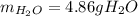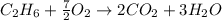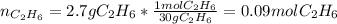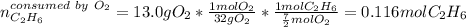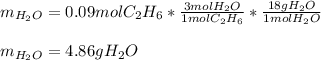Gaseous ethane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . Suppose 2.7 g of ethane is mixed with 13.0 g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Round your answer to significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1

Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2

Chemistry, 23.06.2019 02:00
Which statement is true about the model of the electromagnetic spectrum a: it change the frequencies of light. b: it compare wavelengths of light. c: the color of light waves can be changed using the model. d: the intensities of light waves can be decreased using the model.
Answers: 2
You know the right answer?
Gaseous ethane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water ....
Questions




Mathematics, 04.01.2020 01:31



Mathematics, 04.01.2020 01:31


Mathematics, 04.01.2020 01:31

Business, 04.01.2020 01:31

Mathematics, 04.01.2020 01:31

Mathematics, 04.01.2020 01:31

History, 04.01.2020 01:31



Mathematics, 04.01.2020 01:31