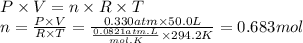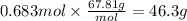Boron trifluoride gas is collected at in an evacuated flask with a measured volume of . When all the gas has been collected, the pressure in the flask is measured to be . Calculate the mass and number of moles of boron trifluoride gas that were collected. Round your answer to significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which part of a feedback mechanism is able to monitor the conditions outside of cells and usually uses nerve cells to relay this information to an intergrating center
Answers: 2

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 23.06.2019 02:10
Detrimental the length of the object shown 1. 97.8mm 2. 97.80 mm 3. 97mm 4. 98mm
Answers: 2
You know the right answer?
Boron trifluoride gas is collected at in an evacuated flask with a measured volume of . When all the...
Questions



Chemistry, 22.10.2020 19:01





English, 22.10.2020 19:01



Mathematics, 22.10.2020 19:01





Social Studies, 22.10.2020 19:01


Advanced Placement (AP), 22.10.2020 19:01