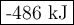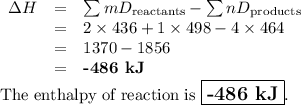Chemistry, 23.06.2020 15:01 dolahghazali76
Use bond energies to calculate ΔHrxn Δ H r x n for the reaction. 2H2(g)+O2(g)→2H2O(g) 2 H 2 ( g ) + O 2 ( g ) → 2 H 2 O ( g )

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Balance this equation: n2 + h2 > nh3, write the following molar ratios: n2 / n2 / nh3 h2 /
Answers: 1

Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2

Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3
You know the right answer?
Use bond energies to calculate ΔHrxn Δ H r x n for the reaction. 2H2(g)+O2(g)→2H2O(g) 2 H 2 ( g ) +...
Questions









Mathematics, 25.04.2020 04:06

Biology, 25.04.2020 04:06