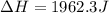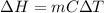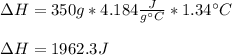Chemistry, 25.06.2020 09:01 christianmcafee
When 200g of AgNO3 solution mixes with 150 g of NaI solution, 2.93 g of AgI precipitates, and the temperature of the solution rises by 1.34oC. Assume 350 g of solution and a specific heat capacity of 4.184 J/g•oC. Calculate H for the following: Ag+(aq) + I- (aq) → AgI(s)

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 11:30
The dashed segment of the plotted experiment in the graph in the l
Answers: 3

Chemistry, 23.06.2019 17:00
During which of the following phases of the moon do we see the left half of the moon as lit? full moon first quarter moon gibbous moon third quarter moon any is greatly : )
Answers: 1

Chemistry, 23.06.2019 22:00
If 15.6 grams of iron (iii) oxide reacts with 12.8 grams of carbon monoxide to produce 9.58 g of pure iron, what are the theoretical yield and percent yield of this reaction? be sure to show the work that you did to solve this problem. unbalanced equation: fe2o3 + co yields fe + co2
Answers: 1
You know the right answer?
When 200g of AgNO3 solution mixes with 150 g of NaI solution, 2.93 g of AgI precipitates, and the te...
Questions


History, 08.05.2021 02:00

Health, 08.05.2021 02:00



Mathematics, 08.05.2021 02:00

History, 08.05.2021 02:00



Physics, 08.05.2021 02:00

Mathematics, 08.05.2021 02:00

Arts, 08.05.2021 02:00

Chemistry, 08.05.2021 02:00


Mathematics, 08.05.2021 02:00

Mathematics, 08.05.2021 02:00


History, 08.05.2021 02:00