

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 23.06.2019 00:00
What does an electron configuration for an atom relate to the atoms placement on the periodic table
Answers: 2

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1
You know the right answer?
Question 3 (11 points)
A gas has a volume of 690.0mL at -15.1°C and 392.0 mmHg. What would the volu...
Questions





Mathematics, 23.11.2020 17:00

World Languages, 23.11.2020 17:00



Mathematics, 23.11.2020 17:00

Mathematics, 23.11.2020 17:00

Social Studies, 23.11.2020 17:00


Mathematics, 23.11.2020 17:00

Mathematics, 23.11.2020 17:00


Mathematics, 23.11.2020 17:00

History, 23.11.2020 17:00


History, 23.11.2020 17:00

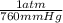 = 0.516 atm
= 0.516 atm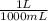 = 0.69 L
= 0.69 L

