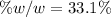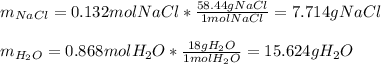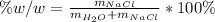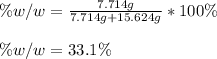Chemistry, 15.07.2020 01:01 datboyjulio21
The mole fraction of NaCl in an aqueous solution is 0.132. What is the weight/weight percent of NaCl in this solution?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Si una estrella no tiene paralaje medible, ¿qué puedes inferir?
Answers: 1

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3
You know the right answer?
The mole fraction of NaCl in an aqueous solution is 0.132. What is the weight/weight percent of NaCl...
Questions

Mathematics, 22.12.2020 02:40

Mathematics, 22.12.2020 02:40


Mathematics, 22.12.2020 02:40

English, 22.12.2020 02:40

Geography, 22.12.2020 02:40


Mathematics, 22.12.2020 02:40







Mathematics, 22.12.2020 02:40

Mathematics, 22.12.2020 02:40




Business, 22.12.2020 02:40