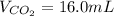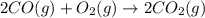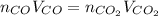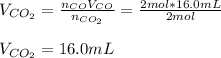Chemistry, 15.07.2020 09:01 westlakebuddy1229
Assuming the same temperature and pressure for each gas, how many milliliters of carbon dioxide are produced from 16 0 mL of CO
2 CO(g) + O2(g) 4, 2 CO2 (g)
Express your answer with the appropriate units.

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 01:30
Ariver current has a velocity of 5km/h relative to the shore, and a boat moves in the same direction as the current at 5 km/h relative to the river. how can the velocity of the boat relative to the shore be calculated?
Answers: 1

Chemistry, 23.06.2019 02:00
What can be done to make a solid solute dissolve faster in a liquid solvent?
Answers: 1

Chemistry, 23.06.2019 06:30
The polarity of an oxygen-hydrogen bond is higher than the polarity of a nitrogen-hydrogen bond, allowing amines to be more soluble than alcohols.
Answers: 3
You know the right answer?
Assuming the same temperature and pressure for each gas, how many milliliters of carbon dioxide are...
Questions



Mathematics, 11.12.2020 01:30




Social Studies, 11.12.2020 01:40


English, 11.12.2020 01:40

Mathematics, 11.12.2020 01:40



Mathematics, 11.12.2020 01:40

Mathematics, 11.12.2020 01:40


Mathematics, 11.12.2020 01:40

Business, 11.12.2020 01:40

Chemistry, 11.12.2020 01:40

History, 11.12.2020 01:40