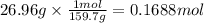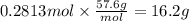Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Where are each of the three particles located within the atom?
Answers: 1


Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3
You know the right answer?
A combination reaction is given below: 3A 2B --5C If compound A has a molar mass of 159.7 g/mole and...
Questions


Computers and Technology, 27.11.2019 23:31


Computers and Technology, 27.11.2019 23:31












Computers and Technology, 27.11.2019 23:31