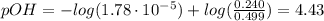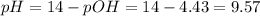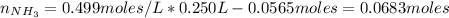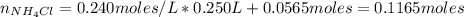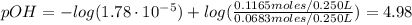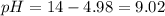Chemistry, 18.07.2020 01:01 DESIRE44030
A buffer solution contains 0.240 M ammonium chloride and 0.499 M ammonia. If 0.0565 moles of perchloric acid are added to 250 mL of this buffer, what is the pH of the resulting solution? (Assume that the volume does not change upon adding perchloric acid.)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Why do sodium and neon have vastly different chemical and physical properties despite having similar atomic masses?
Answers: 2

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2
You know the right answer?
A buffer solution contains 0.240 M ammonium chloride and 0.499 M ammonia. If 0.0565 moles of perchlo...
Questions

Mathematics, 27.02.2020 02:28







English, 27.02.2020 02:28








Mathematics, 27.02.2020 02:28

Social Studies, 27.02.2020 02:29



Mathematics, 27.02.2020 02:29

![pOH = pKb + log(\frac{[NH_{4}Cl]}{[NH_{3}]})](/tpl/images/0708/9694/eb1d2.png)