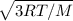Chemistry, 14.08.2020 04:01 AlmightyThadd
A sample of argon gas (molar mass 40 g) is at four times the absolute temperature of a sample of hydrogen gas (molar mass 2 g). Find the ratio of the rms speed of the argon molecules to that of the hydrogen. Assume hydrogen molecule has only translational degree of freedom.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1

Chemistry, 22.06.2019 19:00
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1


Chemistry, 23.06.2019 05:30
Astudent made the lewis dot diagram of a compound as shown. mg is written with two dots shown on its top. an o is written on each side of mg. each o has six dots around it. an arrow is shown from one dot on mg toward the vacant space around the o on the right. another arrow is shown from the other dot on mg toward the vacant space around the o on the left. the title of the art is students lewis dot model. what is the error in the lewis dot diagram? an o atom should transfer all its six electrons to mg because the formula is mgo. both electrons of mg should be transferred to one o atom because the formula is mgo. the electrons should be transferred from each o atom to mg because mg has fewer electrons. the number of dots around mg should be four because it has to transfer two electrons to each o.
Answers: 2
You know the right answer?
A sample of argon gas (molar mass 40 g) is at four times the absolute temperature of a sample of hyd...
Questions

Social Studies, 28.10.2021 19:10




Mathematics, 28.10.2021 19:20

Mathematics, 28.10.2021 19:20



Social Studies, 28.10.2021 19:20


History, 28.10.2021 19:20

Mathematics, 28.10.2021 19:30


Business, 28.10.2021 19:30



Mathematics, 28.10.2021 19:40



Mathematics, 28.10.2021 19:50