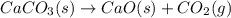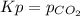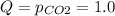Chemistry, 19.08.2020 04:01 Shaness6941
Which direction will the following reaction (in a 5.0 L flask) proceed if the pressure of CO_2(g) is 1.0 atm? CaCO_3(s) rightarrow CaO(s) + C02(g) Kp = 1.9 times 10^-23
a. To the right because Q > K_p
b. To the right because Q < K_p
c. To the left because Q < K_p
d. To the left because Q > K_p

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following pairs of elements belong to the same groupa. h and he b. li and bec. c and pb d. ga and ge
Answers: 1

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1
You know the right answer?
Which direction will the following reaction (in a 5.0 L flask) proceed if the pressure of CO_2(g) is...
Questions

English, 17.12.2020 18:00

Social Studies, 17.12.2020 18:00

Health, 17.12.2020 18:00



Arts, 17.12.2020 18:00


Social Studies, 17.12.2020 18:00


History, 17.12.2020 18:00




History, 17.12.2020 18:00





Mathematics, 17.12.2020 18:00

Mathematics, 17.12.2020 18:00