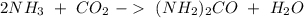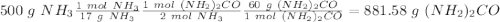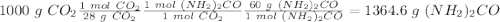Chemistry, 26.08.2020 01:01 davienwatson8
Sea la reacción de síntesis de la urea: 2 NH3 + CO2 → (NH2)2CO + H2O Si tenemos 500 gramos de NH3 y 1000 gramos de CO2 calcular cuál es el reactivo limitante y la cantidad de urea producida.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 2


Chemistry, 22.06.2019 23:30
To find the work done, the force exerted and distance moved are multiplied. a couch is moved twice before you are happy with its placement. the same force was used to move the couch both times. if more work is done the first time it is moved, what do you know about the distance it was moved? a) when more work was done, the couch was moved the same distance. b) when more work was done, the couch was moved less. c) when more work was done, the couch was moved further. d) when more work was done, the couch wasn't moved at all.
Answers: 1

Chemistry, 23.06.2019 05:30
Find the midpoint of a segment with endpoints of 4-3i and -2+7i
Answers: 2
You know the right answer?
Sea la reacción de síntesis de la urea: 2 NH3 + CO2 → (NH2)2CO + H2O Si tenemos 500 gramos de NH3 y...
Questions


English, 28.09.2019 03:30



Biology, 28.09.2019 03:30

Chemistry, 28.09.2019 03:30









Mathematics, 28.09.2019 03:30



Mathematics, 28.09.2019 03:30


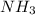 y la cantidad de urea producida es 881.58 g
y la cantidad de urea producida es 881.58 g