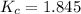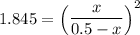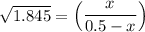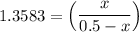Chemistry, 05.09.2020 20:01 Rperez6491
For the reaction:
CO(g) + H2O(g) ⇌ CO2(g) + H2(g)
the value of Kc is 1.845 at a specific temperature. We place 0.500mol CO and 0.500mol H2O in a 1.00L container at this temperature and allow the reaction to reach equilibrium. Determine the equilibrium concentration of all species present in the container.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3


Chemistry, 23.06.2019 09:00
Need ! assume that the variables x and y are directly related. if k = 8, what is the value for each of the following points? be sure and record your data to be used in the following problem. x y k 0.
Answers: 2

Chemistry, 23.06.2019 10:30
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3 2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1
You know the right answer?
For the reaction:
CO(g) + H2O(g) ⇌ CO2(g) + H2(g)
the value of Kc is 1.845 at a specifi...
the value of Kc is 1.845 at a specifi...
Questions


Physics, 06.10.2021 14:00

English, 06.10.2021 14:00

Social Studies, 06.10.2021 14:00

History, 06.10.2021 14:00

History, 06.10.2021 14:00

Mathematics, 06.10.2021 14:00


Mathematics, 06.10.2021 14:00


Mathematics, 06.10.2021 14:00



Spanish, 06.10.2021 14:00

Mathematics, 06.10.2021 14:00

Chemistry, 06.10.2021 14:00



Chemistry, 06.10.2021 14:00

![K_c = \dfrac{[x][x]}{[0.5-x][0.5-x]}](/tpl/images/0743/5235/b80f7.png)
![K_c = \dfrac{[x]^2}{[0.5-x]^2}](/tpl/images/0743/5235/7f3bb.png)