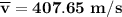Consider a closed container of gas that is a mixture of 30% CO2 and 70% N2 at room temperature 20°C. The gases are in thermal equilibrium with one another. a) Which has the higher kinetic energy, the average CO2 or N2 molecule? b) What is that root-mean-square velocity of a CO2 molecule? For reference, a carbon atom has 6 protons, a nitrogen atom has 7 protons, and an oxygen atom has 8 protons.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1

Chemistry, 23.06.2019 01:00
Animals that reproduce sexually either do it through external or internal fertilization. read the following statement and decide if it is true or false. birds reproduce through external reproduction which is because the female will then be able to protect the egg.
Answers: 1

Chemistry, 23.06.2019 04:10
What does the field of thermodynamics relate to a-changes in nuclear reactions b- changes in energy in systems c changes in molecular structure d changes in atomic properties
Answers: 1
You know the right answer?
Consider a closed container of gas that is a mixture of 30% CO2 and 70% N2 at room temperature 20°C....
Questions

History, 31.08.2019 22:30


History, 31.08.2019 22:30

History, 31.08.2019 22:30

Biology, 31.08.2019 22:30

Mathematics, 31.08.2019 22:30

History, 31.08.2019 22:30


Mathematics, 31.08.2019 22:30


History, 31.08.2019 22:30

Mathematics, 31.08.2019 22:30



Social Studies, 31.08.2019 22:30


History, 31.08.2019 22:30

Mathematics, 31.08.2019 22:30