Chemistry, 15.10.2020 19:01 ShianHagen5
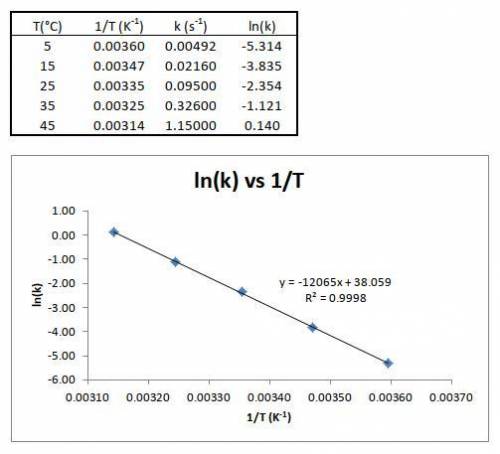
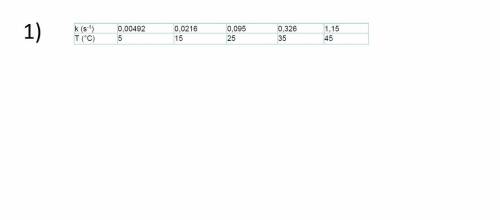
1) The speed constant was determined for the first order decomposition of an organic compound at various temperatures:
Calculate the activation energy for the reaction. Build a graph of Arhenius
2) The hydrolysis of sucrose. in which a sucrose molecule is broken in a glucose molecule and a fructose molecule, is part of the digestive process. Calculate the speed constant for the hydrolysis of sucrose at 35,0 C knowing that k=1,0x10^-3 L mol^-1s^-1 at 37 C and that the activation energy is 108kj mol^-1

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1
You know the right answer?
1) The speed constant was determined for the first order decomposition of an organic compound at var...
Questions


Social Studies, 14.05.2021 21:10


History, 14.05.2021 21:10

History, 14.05.2021 21:10


Chemistry, 14.05.2021 21:10

Mathematics, 14.05.2021 21:10

Mathematics, 14.05.2021 21:10

History, 14.05.2021 21:10

Mathematics, 14.05.2021 21:10

History, 14.05.2021 21:10


Mathematics, 14.05.2021 21:10

Arts, 14.05.2021 21:10

History, 14.05.2021 21:10

English, 14.05.2021 21:10

Mathematics, 14.05.2021 21:10


Mathematics, 14.05.2021 21:10