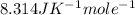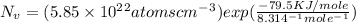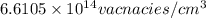Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

You know the right answer?
Calculate the concentration of vacancies in in fcc silver at 250oC given that the activation energyf...
Questions


Mathematics, 04.04.2020 02:23





Mathematics, 04.04.2020 02:23


Chemistry, 04.04.2020 02:23




History, 04.04.2020 02:23







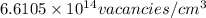
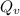 =79.5 kJ/mol
=79.5 kJ/mol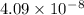 m
m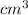
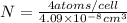
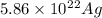 atoms/
atoms/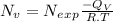
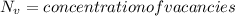
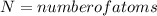
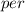
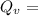 Activation energy for vacancy creation
Activation energy for vacancy creation