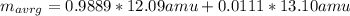Chemistry, 03.11.2020 17:00 mooredollie
An element has two common isotopes. 98.89% of its atoms have an atomic mass of 12.09 amu, whereas the other 1.11% have an atomic mass of 13.10 amu. Using the isotopic composition provided, calculate the average atomic mass of the element.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3
You know the right answer?
An element has two common isotopes. 98.89% of its atoms have an atomic mass of 12.09 amu, whereas th...
Questions

English, 16.08.2019 08:10


Mathematics, 16.08.2019 08:10