
Chemistry, 05.11.2020 16:40 MendesMist
Carbon monoxide and molecular oxygen react to form carbon dioxide. A 50.0 L reactor at 25.0 oC is charged with 1.00 bar of CO. The gas is then pressurized with O2 to give a total pressure of 3.52 bar. The reactor is sealed, heated to 350 oC to drive the reaction to completion, and cooled back to 25.0 oC. Compute the final partial pressure of each gas (in bar).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3


Chemistry, 23.06.2019 03:00
Asample of sea water contains 6.28g of sodium chloride per litre of solution. how many milligrams of sodium chloride would be contained in 15.0ml of this solution?
Answers: 3

Chemistry, 23.06.2019 07:00
Achemist who studies water samples did a demonstration of how to test for lead in water. she added a clear solution of potassium iodide to a clear solution of lead nitrate. then a yellow swirling solid formed in the liquid. what is most likely true about the yellow solid?
Answers: 3
You know the right answer?
Carbon monoxide and molecular oxygen react to form carbon dioxide. A 50.0 L reactor at 25.0 oC is ch...
Questions





Geography, 22.06.2019 12:30

Chemistry, 22.06.2019 12:30



History, 22.06.2019 12:30

English, 22.06.2019 12:30


Biology, 22.06.2019 12:30

English, 22.06.2019 12:30


Mathematics, 22.06.2019 12:30

Mathematics, 22.06.2019 12:30




Chemistry, 22.06.2019 12:30

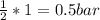 (required)
(required)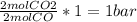 of CO2 2.52 bar O2 (initially) - 0.5 bar (reacted) = 2.02bar O2
of CO2 2.52 bar O2 (initially) - 0.5 bar (reacted) = 2.02bar O2

