
Chemistry, 05.11.2020 18:50 andreanaapollon7593
A compound containing Na, C, and O is found to have 1.06 mol Na, 0.528 mol C, and 1.59 mol O. What is the empirical formula of the compound?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3
You know the right answer?
A compound containing Na, C, and O is found to have 1.06 mol Na, 0.528 mol C, and 1.59 mol O. What i...
Questions

Mathematics, 27.07.2019 20:30


Mathematics, 27.07.2019 20:30

Mathematics, 27.07.2019 20:30


Spanish, 27.07.2019 20:30

Advanced Placement (AP), 27.07.2019 20:30

Computers and Technology, 27.07.2019 20:30

Mathematics, 27.07.2019 20:30

History, 27.07.2019 20:30







Mathematics, 27.07.2019 20:30


History, 27.07.2019 20:30

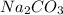 .
.

