Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1
You know the right answer?
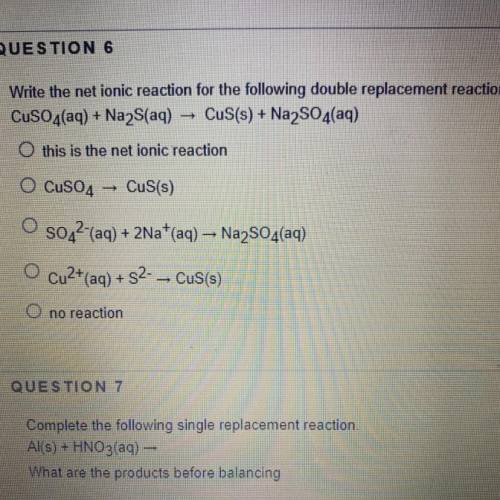
Write the net ionic reaction for the following double replacement reaction:
CuSO4(aq) + Na2S(aq) →...
Questions





History, 09.10.2019 19:20

Computers and Technology, 09.10.2019 19:20

Computers and Technology, 09.10.2019 19:20

Computers and Technology, 09.10.2019 19:20

Computers and Technology, 09.10.2019 19:20

Computers and Technology, 09.10.2019 19:20

Computers and Technology, 09.10.2019 19:20

Computers and Technology, 09.10.2019 19:20

Computers and Technology, 09.10.2019 19:20

Computers and Technology, 09.10.2019 19:20

Computers and Technology, 09.10.2019 19:20



Computers and Technology, 09.10.2019 19:20