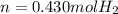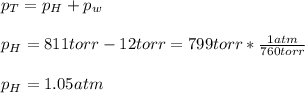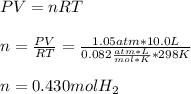Chemistry, 17.11.2020 01:00 alecnewman2002
a sample of hydrogen gas (h2) is mixed with water vapor (h2o (g)). the make sure has a total pressure of 811 torr, and the water vapor has a partial pressure of 12 torr. how many moles of hydrogen gas are present in a 10.0 l mixture at 298k?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
How did planetesmals form planets? a. they broke apart into smaller chunks.b. they collided and stuck together.c. they cooled and pulled ice together.d. they began to rotate.
Answers: 1

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1
You know the right answer?
a sample of hydrogen gas (h2) is mixed with water vapor (h2o (g)). the make sure has a total pressur...
Questions









Mathematics, 14.03.2020 02:40

Mathematics, 14.03.2020 02:40


English, 14.03.2020 02:40

Geography, 14.03.2020 02:40