Chemistry, 20.11.2020 17:00 queenpaige2015
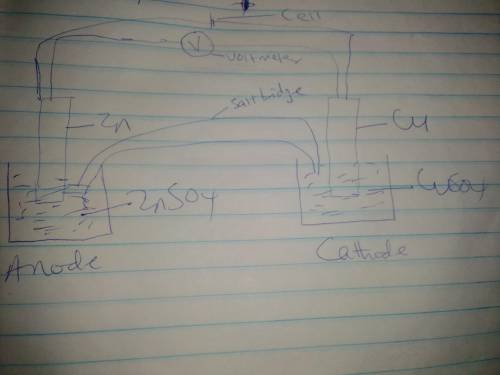
Use the given half reactions to "construct" an electrolytic cell. Zn^2+ + 2 e^>Zn E°cell = -0.76 V Cu^2+ + 2 e^> Cu E°cell = 0.34 V 1. Predict the standard potential of the cell at 298 K. 2. What is the minimum voltage that should be applied to the standard electrolytic cell found in question to cause zn2+ to be reduced to Zn?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3


Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1
You know the right answer?
Use the given half reactions to "construct" an electrolytic cell. Zn^2+ + 2 e^>Zn E°cell = -0.76...
Questions



Mathematics, 01.09.2019 01:30

Mathematics, 01.09.2019 01:30



History, 01.09.2019 01:30

Mathematics, 01.09.2019 01:30

Health, 01.09.2019 01:30



Mathematics, 01.09.2019 01:30



History, 01.09.2019 01:30



Social Studies, 01.09.2019 01:30

Mathematics, 01.09.2019 01:30