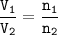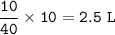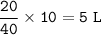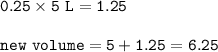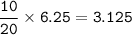The chemical equilibrium is given:
2NO (g) + O2(g) <—>2N O2, (g)
In a container...

Chemistry, 25.11.2020 14:00 shartman22
The chemical equilibrium is given:
2NO (g) + O2(g) <—>2N O2, (g)
In a container of volume 10L is in equilibrium a mixture consisting of 10 mol NO (g), 10 mol O2, (g) and 20 mol NO2, (g).
The volume of the container changes under constant temperature and after the restoration of equilibrium the amount of NO has increased by 25%. Calculate the volume change in L.
How do I find the change in volume?
P. s sorry if my English isn’t perfect.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 23.06.2019 08:00
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 3

You know the right answer?
Questions

Biology, 24.03.2020 05:25