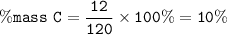Chemistry, 29.11.2020 01:20 axell13965
What percentage of Carbon (C) is present in one molecule of
Dichlorodifluoromethane (CF2Cl2), commonly known as Freon, an anti-freezing agent
used in automobiles.
Please round everything to whole numbers for this question. Use the following
masses in your calculations:
C = 12
F = 19
Cl = 35

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 23.06.2019 03:00
What happens in the particles of a gas when the gas is compressed
Answers: 1
You know the right answer?
What percentage of Carbon (C) is present in one molecule of
Dichlorodifluoromethane (CF2Cl2), commo...
Questions

Social Studies, 03.02.2020 11:53

Mathematics, 03.02.2020 11:53


Mathematics, 03.02.2020 11:54

Mathematics, 03.02.2020 11:54


English, 03.02.2020 11:54


English, 03.02.2020 11:54


Mathematics, 03.02.2020 11:54


Social Studies, 03.02.2020 11:54

Mathematics, 03.02.2020 11:54