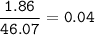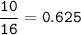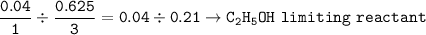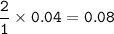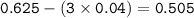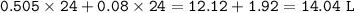Consider the following unbalanced equation:
C2H5OH(g) + O2(g) → CO2(g) + H2O(l)
1.86 g of et...

Chemistry, 03.12.2020 14:00 Seumas9307
Consider the following unbalanced equation:
C2H5OH(g) + O2(g) → CO2(g) + H2O(l)
1.86 g of ethanol reacts with 10.0 g of oxygen. What is the total volume of gas present (in L) after the reaction is complete, assuming the reaction takes place at 1.00 atm and 25°C?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 23.06.2019 06:00
What are the coefficients to balance the following equation? ba+br2=babr2
Answers: 2

Chemistry, 23.06.2019 07:40
Which of the following has expanded our knowledge of the universe beyond our solar system the most? a. manned space travel b. the hubble space telescope c. the pioneer and voyager missions d. the international space station
Answers: 3
You know the right answer?
Questions


Mathematics, 19.12.2020 14:00

Mathematics, 19.12.2020 14:00

English, 19.12.2020 14:00

Mathematics, 19.12.2020 14:00


Chemistry, 19.12.2020 14:00

Computers and Technology, 19.12.2020 14:00

English, 19.12.2020 14:00


Social Studies, 19.12.2020 14:00


Mathematics, 19.12.2020 14:00

Arts, 19.12.2020 14:00

Mathematics, 19.12.2020 14:00

Mathematics, 19.12.2020 14:00


Mathematics, 19.12.2020 14:00

Mathematics, 19.12.2020 14:00

English, 19.12.2020 14:00